SUMMARY
Enovacom’s vision
Whether we are talking about prevention, diagnosis, or health monitoring, or are in a healthcare facility or at home, we all eventually get our vital signs checked: blood pressure, oxygen saturation, pulse rate, etc. This reality is even more evident with modern medical devices that differ from traditional care patterns and blend into our daily lives, particularly by integrating with our watches and smartphones.
In short, we are generating health data and will continue to do so. For Enovacom, the vision is to optimize the automatic collection of vital signs to promote a broader use of this data in the long term.
Beyond patients, the same is true for caregivers who strive to improve their quality of work life. Consider the benefit of automatic data entry for caregivers, helping them focus on patients while ensuring data is both accurate and reliable.
But how can we ensure biomedical devices and patient records interconnect? Medical device manufacturers have created multiple communication libraries and business software editors have developed several interfaces.
Biomedical interoperability takes on its full meaning in this context. This interconnection and its benefits are only possible by investing in a global interoperability strategy.
By focusing first on the final beneficiaries, namely information systems directors, caregivers, and patients, Enovacom is investing in biomedical interoperability.
We prioritize saving time, ensuring data traceability and improving quality of work life to best organize each patient’s medical care.
What is biomedical interoperability?
Biomedical interoperability refers to the overall ability of a hospital information system to collect, integrate and exchange data from medical devices.
We can define biomedical interoperability using three core pillars:
- Collecting and sharing patient data.
- Improving health data reliability and traceability.
- Standardizing caregivers’ practices.
Collecting and sharing patient data
This is the most technical aspect. The objective is to link the medical device to a third-party system, such as a computerized care record, by adapting to their respective language and communication protocol.
In practice, systems collect data from a medical device through communication libraries specific to each one. Enovacom has chosen to foster the sharing of these vital signs with the provision of a standardized message in HL7 format.
In addition, the Integrating the Healthcare Enterprise (IHE) initiative makes recommendations on the use of the HL7 format so that all those involved in biomedical interoperability can understand and apply this standard in a uniform way.
What is a message in HL7 format?
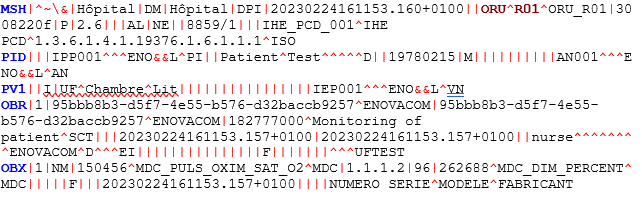
In this message, you will find information on the identity of the patients who came to the healthcare facility and their vital signs.
Here: M. Test Patient, born 02/15/1978, shows a 96% oxygen saturation reading.
Improving health data reliability and traceability
Biomedical interoperability plays a crucial role in improving health data reliability and traceability.
- Patient data integrity: the information is sent in an accurate and consistent manner, without changing throughout its flow, using common standards and protocols.
- Accurate management and interpretation: by allowing system compatibility, the data is available in healthcare professionals’ business software. Aside from saving time, it also enables comprehensive interpretation by making all the data available in one place. This improves diagnoses and makes medical decisions easier.
- Auditability and traceability: interoperability in the healthcare system enables the tracking and tracing of medical information at every stage of their flow. The data can be saved with information on its origin, date, time, and edits, facilitating data traceability and auditing. This is a major challenge for hospitals today on the grounds of responsibility, regulatory compliance (GDPR in Europe, health data hosting, etc.) and the search for errors or data manipulation.
- Identity monitoring: a key element of patient safety in a healthcare facility, identity monitoring consists of managing and verifying an individual’s identity at each stage of their care so they receive the proper care. Biomedical interoperability increases confidence in information accuracy and reliability by ensuring precise correlation of medical data with the patients’ identity.
Standardizing caregivers’ practices
Biomedical interoperability goes beyond its purely technical dimension. In fact, understanding how users interact with medical devices and the complexity of the systems they face is crucial to addressing the critical issue of data exploitation from medical devices.
Considering the heterogeneity of medical devices and care records, it is essential to consider simplifying practices through standardization. The goal is to achieve biomedical interoperability that is independent of medical devices and care records from the users’ perspective, as is the case with data flows.
In this context, two major approaches emerge:
- The first, dubbed “location-centric,” is based on the knowledge of the location of patients and medical devices by different systems. Data sent by a medical device from a specific place is automatically attached to the IT record of the patient known in that area.
The main advantage of this is to enable the increase of the parameters without action from the caregiver. This saves the most time.
In return, it is crucial to pay close attention to applying this solution. Moreover, this method only works for fixed medical devices.
- The second, dubbed “patient-centric,” is based on a logical link between patients and medical devices. This link, supported by biomedical interoperability, is used from the medical device, the care record or an external (agnostic) system.
The key benefit of this work method is that the right data is always associated with the right patient. On the other hand, the user must initiate the link.
Medical device interoperability therefore has the role of standardizing medical device data and transmitting it to the target application. The goal is to simplify access to patient data by organizing upstream processes, thereby improving quality of care, patient safety and medical decision-making.
In short, biomedical interoperability can improve quality of work life by reducing the mental load of transcribing data or the requirement of understanding multiple systems, and it can ensure that the right data is always linked to the right patient.

The importance of biomedical interoperability
MULTIPLE SYSTEMS
Biomedical interoperability centralizes the management and oversight of the data produced by these systems.
COMPLEX DATA
Biomedical interoperability automates the recovery and sharing of biomedical data.
RELIABILITY AND SAFETY
Biomedical interoperability administers data from a system to support identity monitoring.
FACILITATING WORK
Biomedical interoperability saves time and reduces caregivers’ mental load.
How to implement a biomedical interoperability strategy
A biomedical device interoperability project involves several key steps:
- • Identifying, presenting and defining the responsibilities of all those involved in the project
- • Validating the project’s functional scope regarding the needs expressed by the care services.
- • Validating the project’s technical scope and the associated constraints (updates, additional equipment, etc.).
- • Validating the projected deployment schedule.
- • Providing an installation prerequisite document to customers.
This initial vision of a global biomedical interoperability project makes it possible to draw up a project management plan and set up a detailed schedule with associated risk management.
Multiple stakeholders must be involved in the project. The caregivers define their day-to-day workflow; the Information Systems Department ensures the integration of biomedical interoperability into their global strategies; and biomedical engineers support the management of their biomedical devices.
Focus on the rollout phase
This phase is crucial because it stems from the scoping of the upstream project. Depending on the project’s scope, functional objectives, and the caregivers’ organization, Enovacom will choose a specific deployment plan to adapt to the institution’s needs.
1. Biomedical interoperability platform installation
The editor installs and configures its solution using the following steps:
- Install the solution.
- Install plugins and additional hardware, as required.
- Technical configuration of the various systems present to validate the correct point-to-point communication.
A set of deliverables must make it possible to precisely trace the actions carried out during these steps, and include installation, functional and technical reports.
2. Functional configuration phase
During this phase, the solution is configured to meet the expectations of the care structure and its users through the following stages:
- Configure medical devices, if necessary.
- Implement use workflow (particularly in terms of identity monitoring).
- Implement data management rules, e.g. sampling.
- Transmit and display data in the computerized patient record.
- End-to-end unit test.
Functional and technical reports must be updated and forwarded to the various stakeholders to ensure optimal flow of operations.
3. Integration phase
The solution is used in real-world conditions and a playbook established to ensure all expected features have been delivered.
4. Project production and monitoring
The interface goes live after integration validation. Users can then take full advantage of the new features. Administrators are trained. The editor switches to a support role and the process is communicated to the client.



